Buffering System In The Body
Buffering system in the body. The carbonatecarbonic acid buffer the phosphate buffer and the buffering of plasma proteins. One is aerobic requires oxygen. Two are anaerobic no oxygen required.
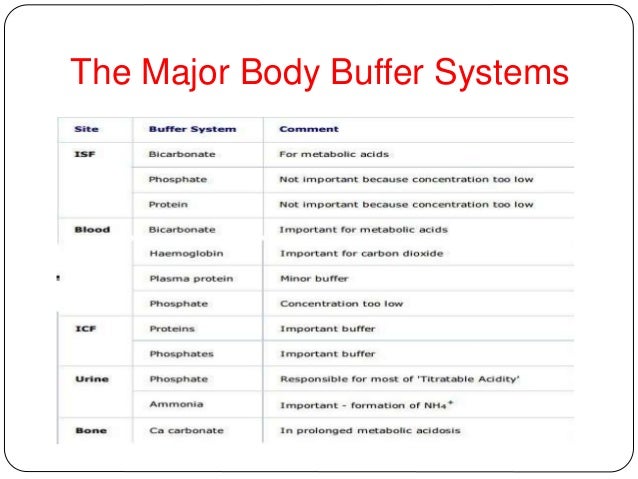
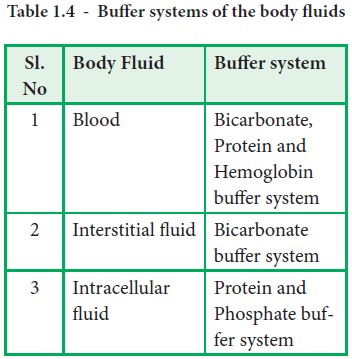
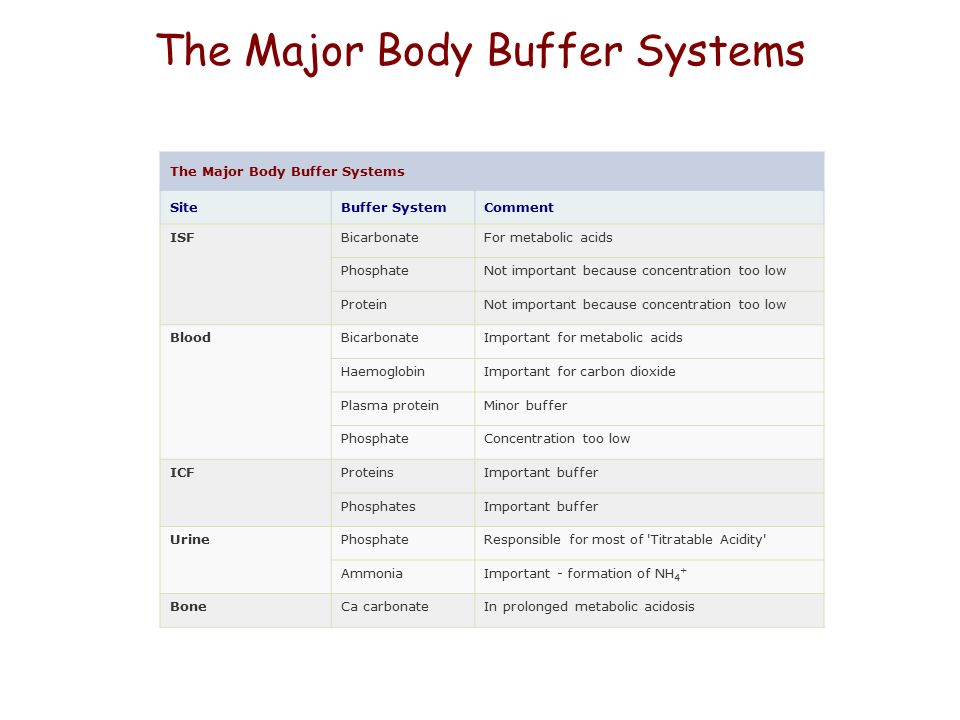
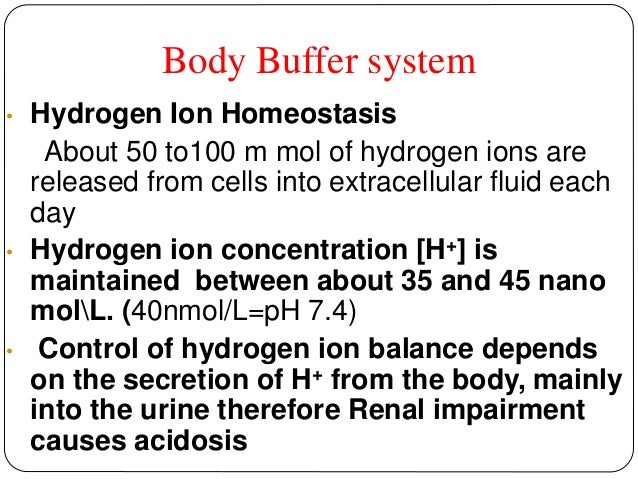
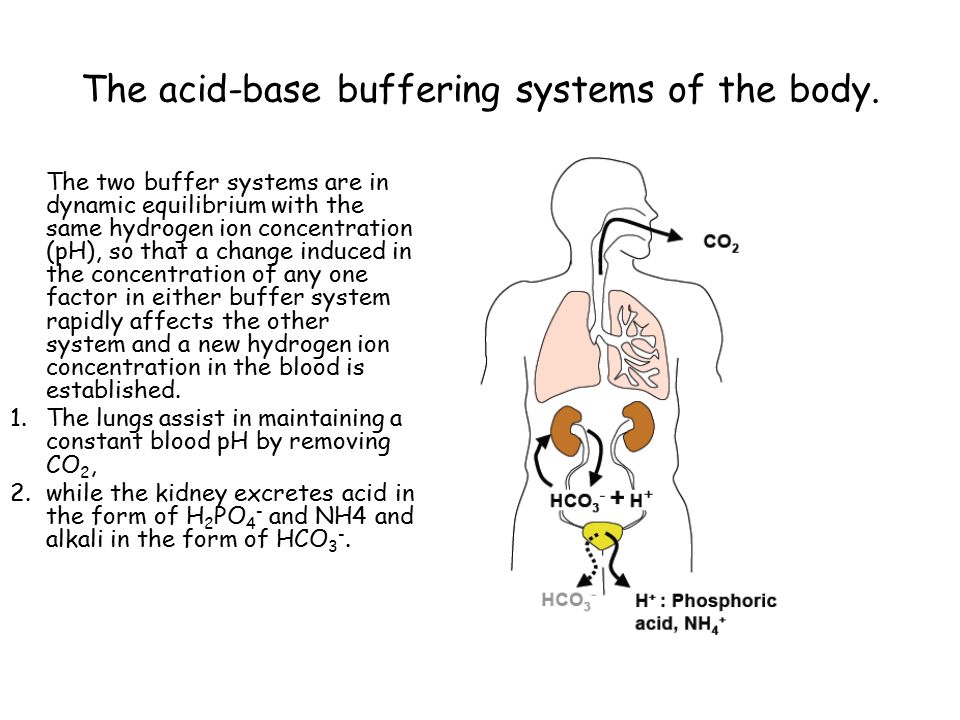
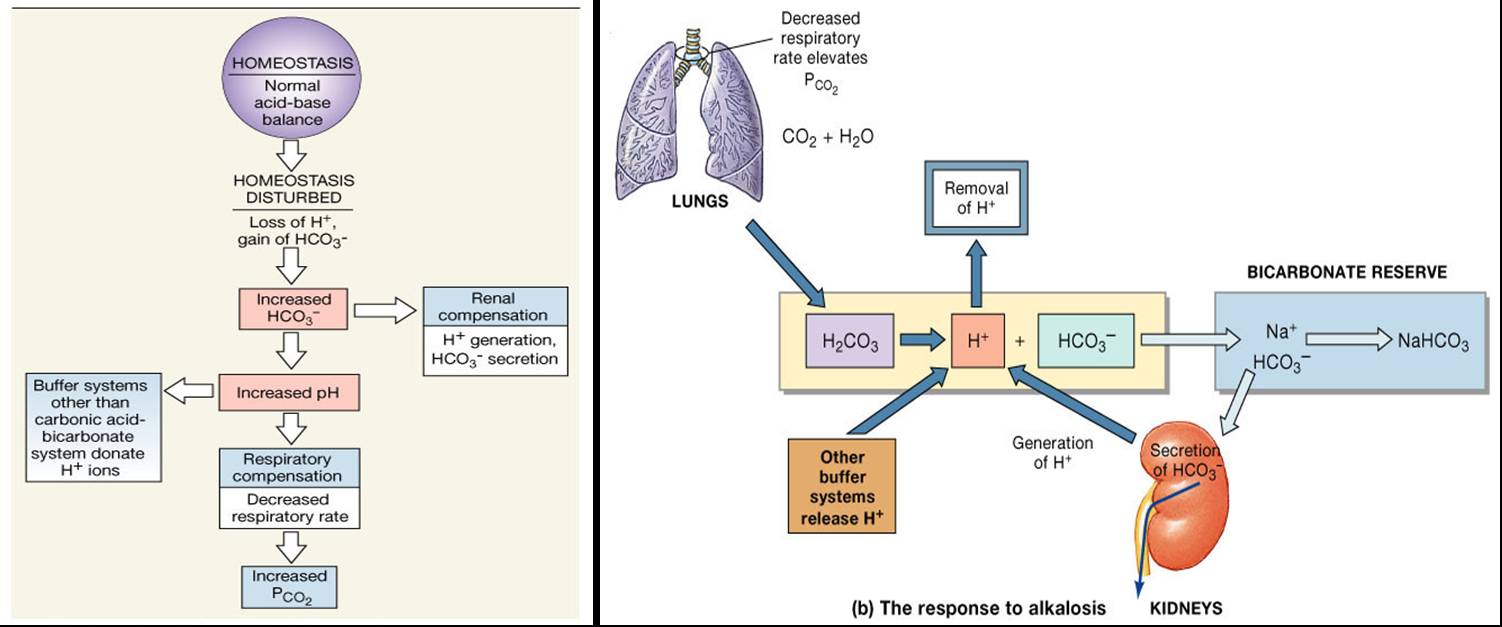
Body Buffer system Hydrogen Ion Homeostasis About 50 to100 m mol of hydrogen ions are released from cells into extracellular fluid each day Hydrogen ion concentration H is maintained between about 35 and 45 nano molL. The respiratory tract can adjust the blood pH upward in minutes by exhaling CO 2. The other two main buffering systems in the blood and circulating cells are phosphates and the bicarbonate-carbonic acid buffer system.
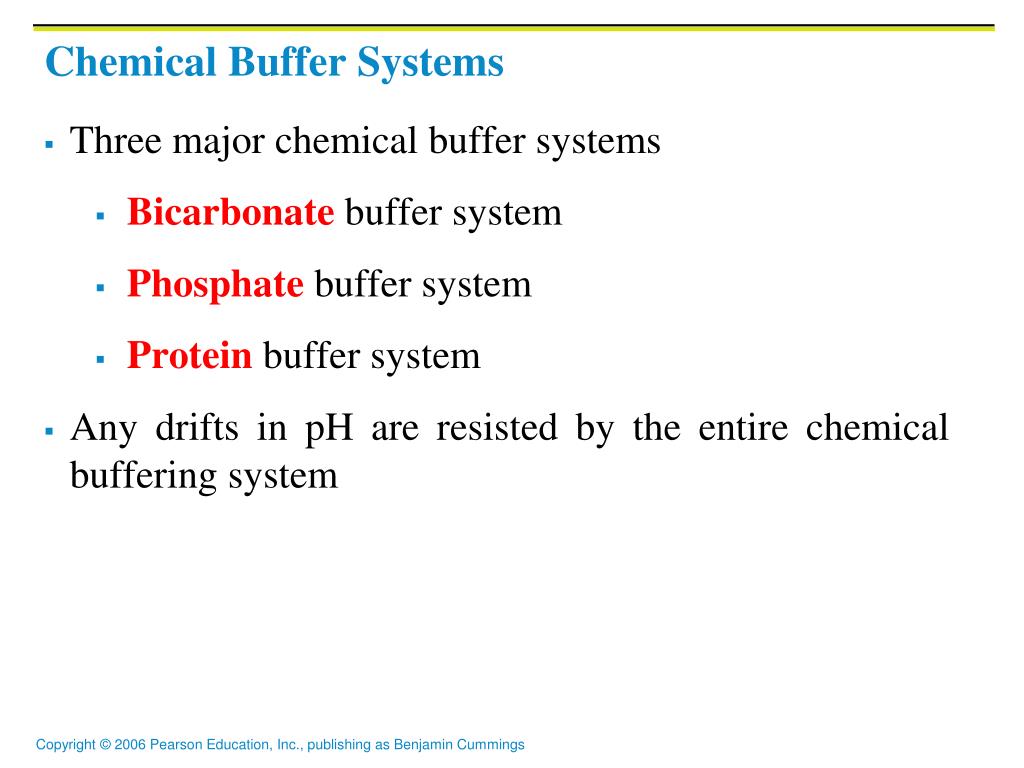
The bodys chemical buffer system consists of three individual buffers. 3 and carbon dioxide CO 2 in order to maintain pH in the blood and duodenum among other tissues to support proper metabolic function. There are three main systems that produce cellular ATP.
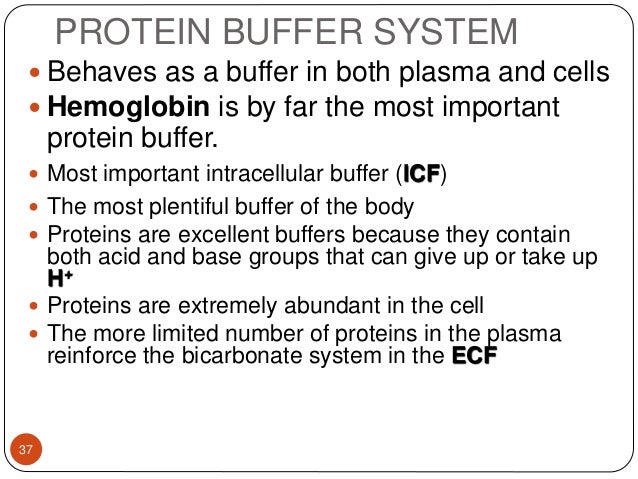
The most important physiological buffers in the body are the bicarbonateCO 2 system the large anion complexes such as plasma proteins and phosphates and hemoglobin in cells. Buffering in blood is crucial to our survival. The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a.
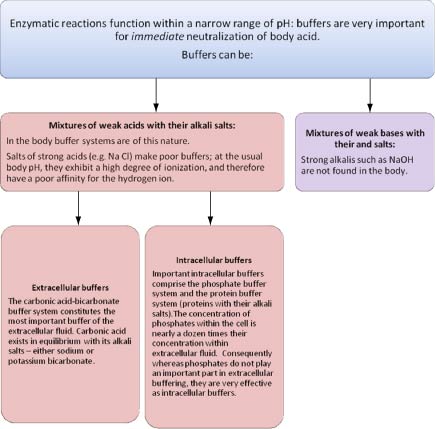
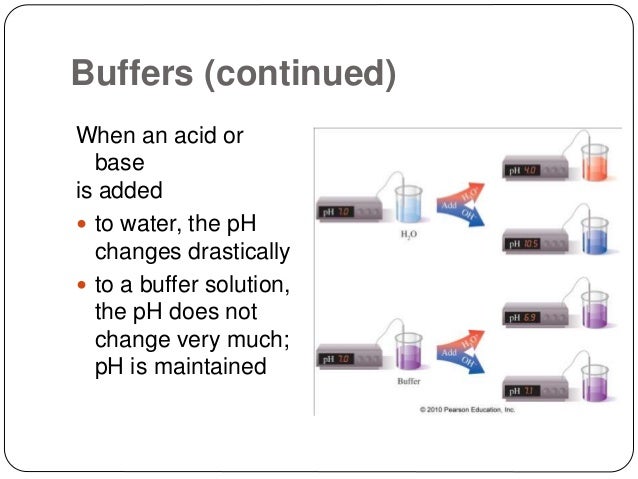
A buffer system exists to help neutralize the blood if excess hydrogen or hydroxide ions are produced. This optimal buffering occurs when the pH is within approximately 1 pH unit from the pK value for the buffering system ie when the pH is between 51 and 71. A buffer solution is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid.
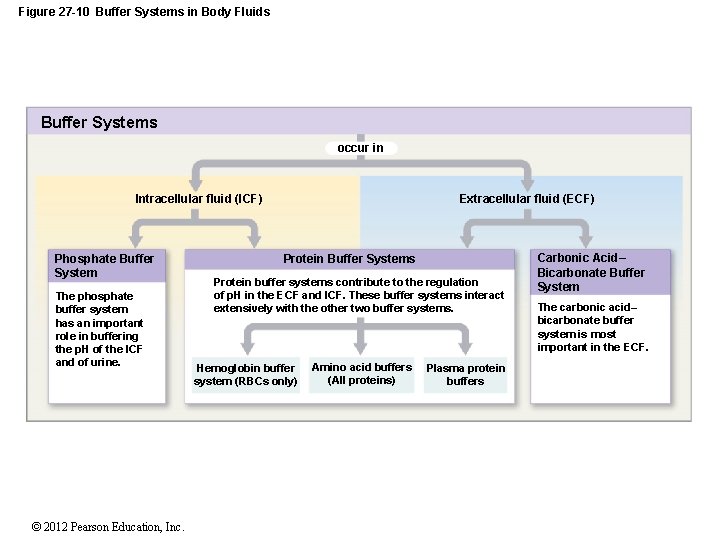
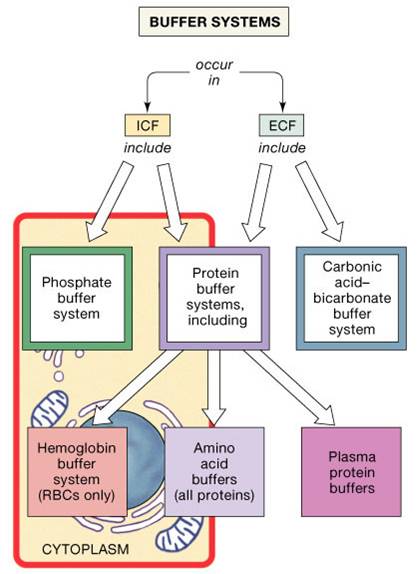
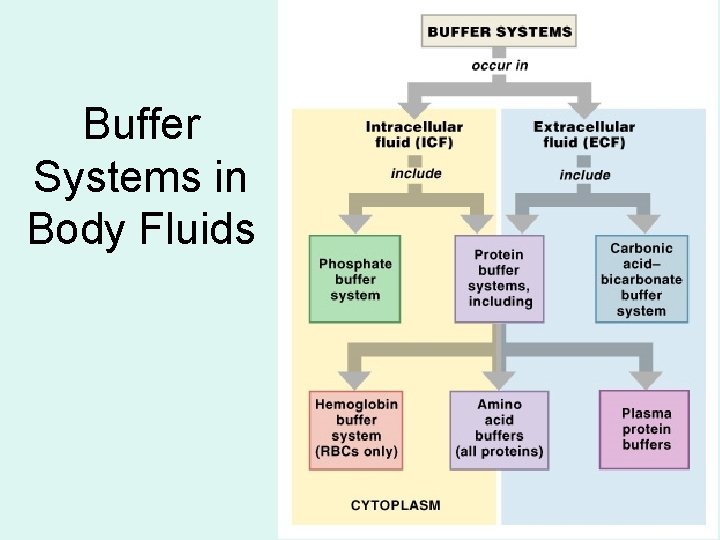
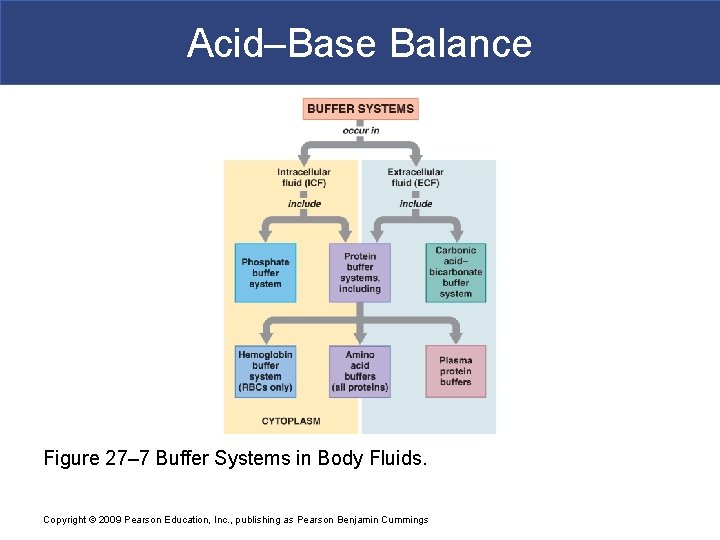
The buffer systems functioning in blood plasma include plasma proteins phosphate and bicarbonate and carbonic acid buffers. Their functionality is mainly intracellular focused and include haemoglobin Hb. Therefore the addition of protons to the blood due to strenuous exercise may be too great for the buffer alone to effectively control the pH of the blood.
While the third buffer is the most plentiful the first is usually considered the most important since it is coupled to the respiratory system. The phosphate buffer system consists of acidic phosphate ions and alkaline phosphate ions that work to neutralize pH.
In all of these the essential reaction is.
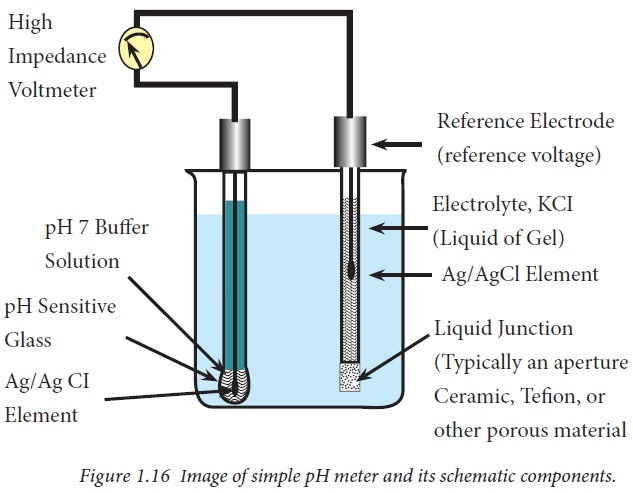
The bodys chemical buffer system consists of three individual buffers out of which the carbonic acid bicarbonate buffer is the most important. Body Buffer system Hydrogen Ion Homeostasis About 50 to100 m mol of hydrogen ions are released from cells into extracellular fluid each day Hydrogen ion concentration H is maintained between about 35 and 45 nano molL. H HCO3 H2CO3 CO2 H2O. The respiratory tract can adjust the blood pH upward in minutes by exhaling CO 2. Catalyzed by carbonic anhydrase carbon dioxide CO 2 reacts. The bodys acid base balance is tightly regulated to keep the arterial blood pH between 738 and 742. 3 and carbon dioxide CO 2 in order to maintain pH in the blood and duodenum among other tissues to support proper metabolic function. However the normal blood pH of 74 is outside the optimal buffering range. Buffering Systems in the Human Body.
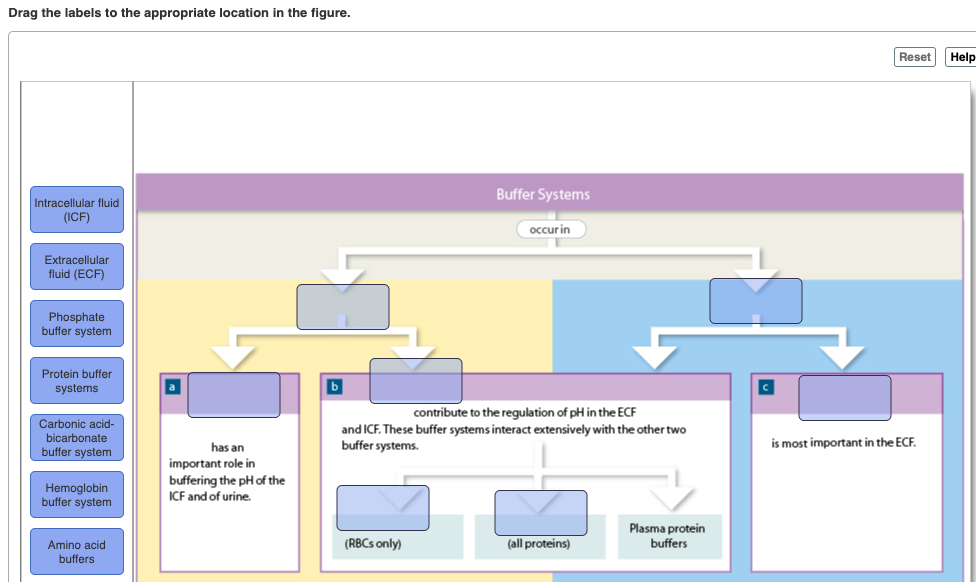
Buffer Systems in the Body. Buffer Systems in the Body. The three primary buffering systems are the protein buffer system the carbonic acid-bicarbonate buffer system and the phosphate buffer system. Buffers and Their Physiological Importance. Buffer Systems in the Body. The bodys acid base balance is tightly regulated to keep the arterial blood pH between 738 and 742. A buffer system in the human body is an interaction between a weak acid-base conjugate pair that keeps the body at the proper pH.





















Posting Komentar untuk "Buffering System In The Body"